3 Top Biotech News Of The Week (8 October 22) - BMS, AUTL, DNA, Svante Pääbo discovery

1) Bristol Myers Squibb (BMS) enters into 2 licensing deals with SynethX and Autolus Therapeutics (AUTL)
Bristol Myers Squibb (NYSE: BMS) entered into 2 licensing deals with SynethX Inc and Autolus Therapeutics (NASDAQ: AUTL).
The first deal is with SynethX which will focus on discovering molecular glue degraders. Synethx has developed the ToRNeDO platform for discovering molecular glue-based protein degraders using pre-specified E3 ligases and neosubstrates of interest.
Molecular glue degraders are compounds that allow interactions between a target protein and an E3 ubiquitin ligase causing the degradation of the target protein via proteasomal degradation.
Apart from the molecular glue technology, there is another protein degradation technology called Proteolysis Targeting Chimeric (PROTAC). A PROTAC is composed of a ligand that binds to the E3 ubiquitin ligase and a ligand that binds to the target protein through a linker. The binding induces polyubiquitination and proteasomal degradation of the target proteins.
In essence, both these technologies work similarly but there are subtle differences between the two technologies which we will cover in the future.
The image below provides a visual example of both types of protein degraders.
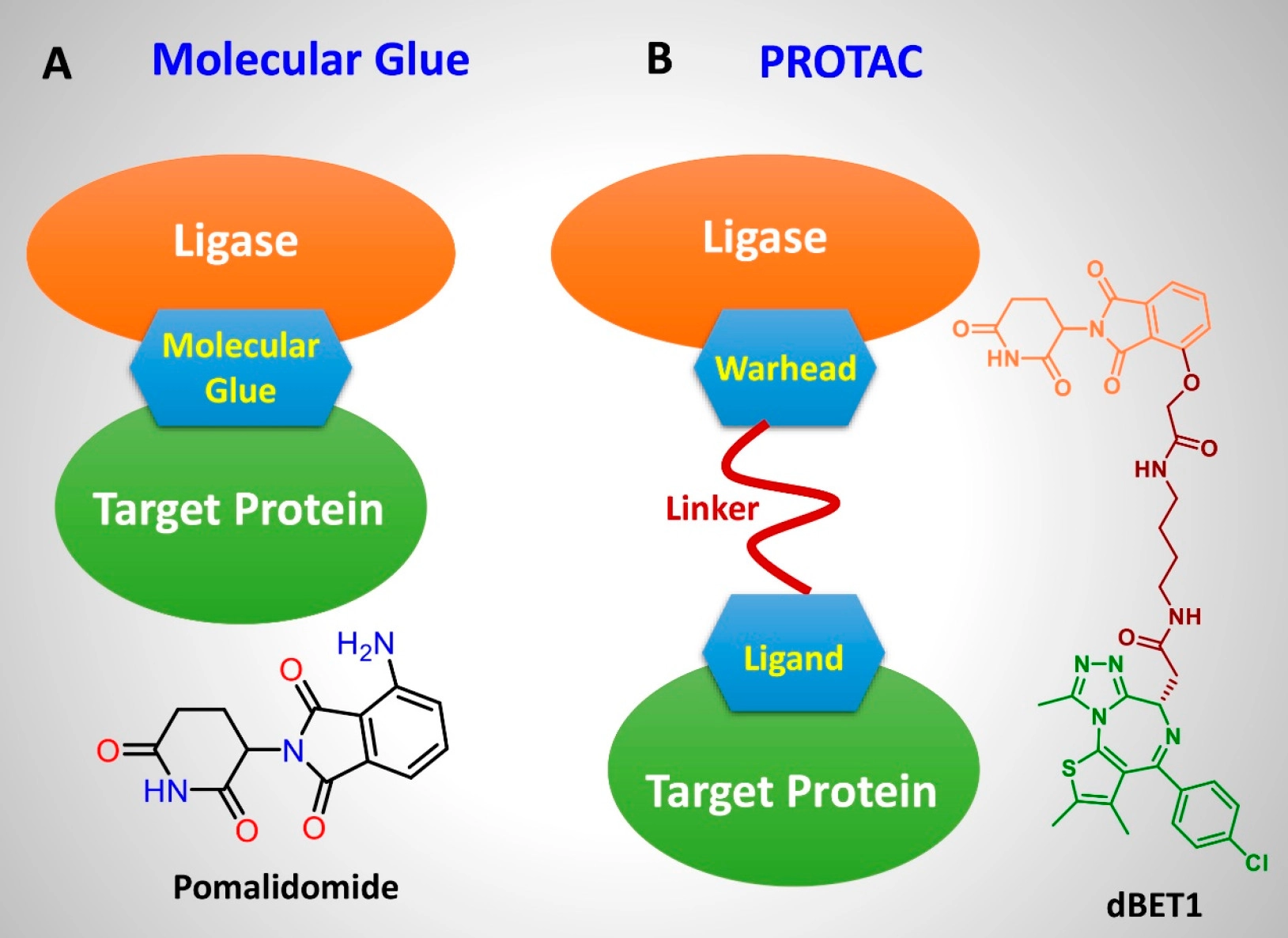
Targeted protein degradation (TPD) is an area in drug discovery seeing increased interest recently because of its potential to use small molecules to fully destroy a disease-causing protein.
SyntheX will receive a combined upfront of cash and investment. SyntheX is also eligible for up to $550 million in performance-based milestone payments, as well as royalties on global net sales of products.
The second collaboration is with Autolus Therapeutics (AUTL). This agreement provides BMS with access to incorporate Autolus’ proprietary RQR8 safety switch into an initial set of selected cell therapy programs on a target-by-target basis for the treatment of cancer, with an option for Bristol Myers Squibb to incorporate the RQR8 safety switch in additional cell therapy programs beyond the initial set of selected programs.
Cell therapies are known to cause toxic side effects, such as cytokine release syndrome and brain toxicity. As such, safety switches, such as Autolus’ RQR8 provide a possible method of limiting these side effects by placing limits on T-cell production.
Autolus’ proprietary RQR8 switch is administered with Genetech and Biogen’s Rituxan (rituximab). Once dosed, rituximab binds to the engineered CD20 epitopes on the surface of the cell therapy and triggers selective cell death via a suicide gene. The schematic below explains how this technology works
Autolus will receive an upfront payment for access to the RQR8 safety switch for the initial set of cell therapy programs with the potential for near-term option exercise fees and development milestone payments. In addition, Autolus would be entitled to receive royalties on net sales of all Bristol Myers Squibb cell therapy products that incorporate the RQR8 safety switch.
2) Gingko Bio works (DNA) goes shopping again
Ginkgo Bioworks (NYSE: DNA) announced 2 tuck-in acquisitions of Circularis and Altar. This comes on the heels of Gingko's acquisition of Zymergen in July, you can read about that here.
Circularis is a biotechnology company with a proprietary circular RNA and promoter screening platform. Circularized RNA lives longer in cells, improving its robustness as a potential therapeutic modality.
You can read about the basics of RNA here - a quick overview of RNA, mRNA, and RNA modalities. And about circular RNA technology in our previous posts which can be found here - 2 companies innovating to overcome the short half-life of mRNA, Orna Therapeutics, and Orbital Therapeutics.
In addition, the Circular platform allows ultra-high-throughput screening of promoters and other enhancers. Ginkgo has expanded its work in cell and gene therapy in recent years and has forged multiple partnerships with Biogen, Selecta Biosciences, Moderna, and Aldevron in this space.
As such, the Circularis platform will provide Gingko with the capability to rapidly identify novel promoters with appropriate strength and tissue-specificity. Overall, the platform will enable new solutions across bioproduction, RNA therapeutics, cell therapy, and gene therapy partnerships and will be a great addition to Gingko's capabilities.
The second acquisition was for Altar, a French biotechnology company that has developed a proprietary adaptive evolution platform.
Gingko is no stranger to Altar's technology as they have collaborated in the past. A fleet of Altar's automated adaptive laboratory evolution (ALE) instruments will be integrated into Ginkgo's Foundry.
The use of adaptive evolution is a go-to strategy when engineering microorganisms for improved growth under unfavorable conditions such as inhibitory concentrations of a target end product or prohibitively high temperatures. These modifications are better derived by evolutionary pressures rather than genome editing as multiple changes in the organism's genome would probably be required.
Nikos Reppas, Senior Director, Foundry Technology at Ginkgo Bioworks commented, " As the range of programs we work on continues to expand, it is imperative that we have the best tools in rational design as well as the ability to leverage the inherent diversity and creativity that emerges from evolutionary processes".
The incorporation of Altars equipment will enable the efficient evolution of targeted microorganisms and will serve customers across multiple verticals such as food and beverage, biofuels, biomaterials, cosmetics, animal health, human health applications, and others. This addition will improve Gingko's offering to its clients and will help to further build up knowledge in solving microorganism optimization which is essential for the growing bio revolution.
3) And the Nobel Medicine Prize goes to...
And the Nobel medicine prize goes to..... Swedish geneticist Svante Pääbo.
Swedish geneticist Svante Pääbo won the 2022 Nobel Prize in Physiology or Medicine on Monday. He was recognized for his discoveries concerning the genomes of extinct hominins and human evolution which underpin our understanding of how modern-day humans evolved from extinct ancestors. His research contributed to our understanding of human development.
Our closest extinct relatives were the Neanderthals. But in 2008, another human relative was discovered when Svante Pääbo studied a bone from the little finger of a human found in the Denisova Cave in Siberia, and it was given the name Denisova. The DNA from the finger bone showed its genetic sequence to be distinct from that of Neanderthals and Homo sapiens.
The last forty thousand years have been unique in human history, in that we are the only form of humans around.
But how did he do it?
After death DNA begins to break down and mix with DNA from other organisms, such as bacteria. Thus, in human remains that are thousands of years old, there is very little DNA left to analyze. When researchers handle such remains today, there is also a great risk of their DNA contaminating the samples. You can read more about the basics of DNA here.
One of the first things Svante Pääbo developed was an advanced method for handling and analyzing mitochondrial DNA from a 40,000-year-old piece of bone. He then became the first to sequence the genome of our closest known extinct relative. This led to the establishment of an entirely new field of research: paleogenomics.
You can read our article on the different applications of DNA sequencing to understand its broad current and future applications.
Through his pioneering research, Svante Pääbo has been able to demonstrate the genetic differences between Homo sapiens and our closest extinct relatives.
For some context, Denisova mitochondrial DNA differs from that of modern humans by 385 bases (nucleotides) out of approximately 16,500, whereas the difference between modern humans and Neanderthals is around 202 bases. In comparison, the difference between chimpanzees and modern humans is approximately 1,462 mtDNA base pairs.
So why is this important?
The sequencing of the genome of Neanderthals and Denisova allows medical researchers to compare their genomes with modern-day humans. These comparisons could then lead to the discovery of new therapeutics or shed light on diseases and potential treatments for them faced by modern-day humans.
Scientists have already started looking in this direction to get new ideas and develop new therapeutics based on the genomes of our extinct relatives. The day is not far when we can say, “Our ancestors saved us”.
If you liked our article, subscribe to our newsletter to receive our latest articles directly in your inbox. The subscribe button can be found at the corner of the page. We will appreciate your support, or hit the follow button on twitter or Linkedin.
Disclaimer: All opinions shared in this article are the opinions of the authors and do not constitute financial advice or recommendations to buy or sell. Please consult a financial advisor before you make any financial decisions. The authors do not hold positions in any of the mentioned securities.
