Exploring Drug Modalities: Small Molecules, Biologics, RNA, Cell & Gene Therapy
Multiple different drug modalities are used as therapeutics for the treatment of different diseases or illnesses. In this post, we will look at the different therapeutic modalities that exist in the pharma/biotech world.
Drugs usually fall under 4 different types of modalities:
1) Small molecule
2) Biologics
3) Cell therapy & gene therapy
4) RNA therapy (Nucleic acid therapeutics)
The image below gives you a sense of the size, complexity, and cell permeability.
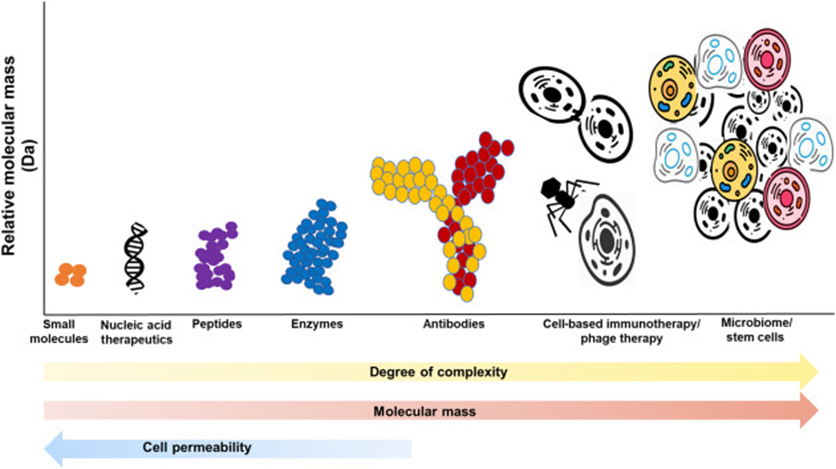
The early days of drug development were mostly concentrated around small molecule drugs as they are easier to discover and develop. But in the last decade, Nucleic acid and antibody therapies have seen increased approvals.
Due to the complexities associated with newer forms of therapeutics, they were difficult to generate and test in the past. But as technology has improved and costs have come down, these more complex modalities can now be developed and tested.
As we look to the next decade (2023 and beyond) we will notice that cell therapies, gene therapies, and advanced biologics will see an increasing number of approvals. The increased complexities in developing these assets mean that they will cost more to develop which will be reflected in their high price tags.
With that, let us look at each modality to get a better understanding.
1) Small molecule (SM) drugs
Small-molecule drugs have been the pillars of traditional medicine and played an important role in shaping the world we live in.
Small molecule drugs have a low molecular weight (small size), allowing them to penetrate cells easily. These compounds work by targeting key processes in molecular pathways to achieve their desired outcome. This could be by inhibiting intracellular enzymes, or by facilitating or blocking signal transductions. The images below explain these different mechanisms of action.
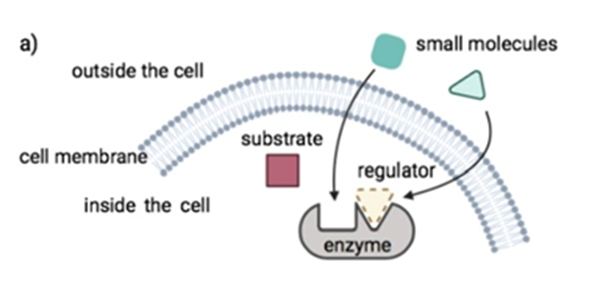
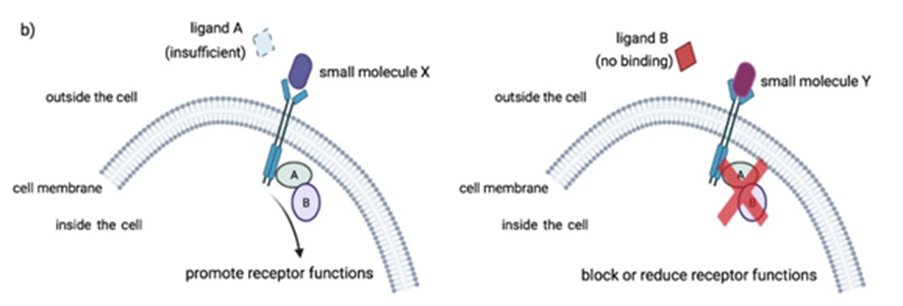
Small molecule drugs are usually developed from leads derived from rational drug design or isolated from natural resources.
A classic example is the wonder drug penicillin. Penicillin, derived from the Penicillium fungi is the natural small-molecule drug that became the first mass-produced antibiotic in the 1940s. Penicillin successfully reduced the death rate by bacterial-related pneumonia to less than 1% during World War II.
Another example of a synthetic small-molecule drug is aspirin. Aspirin has been one of the most popular pharmaceutical agents of the past one hundred years and is a synthetic derivative of the natural substance salicylic acid. Aspirin works by inhibiting the cyclooxygenase enzyme 1(COX 1) and cyclooxygenase enzyme 2 (COX 2), thereby preventing prostaglandin synthesis which causes inflammation and pain.
2) Biologics
Although small-molecule drugs have dominated the pharmaceutical industry since the beginning of modern medicine, we seem to be approaching the era of biologics.
Antibodies make up a large class of biologics. As defined by the FDA, biologics also include other proteins, gene-based therapies, cellular products, vaccines, blood, etc.
For example, insulin has been an essential life-saving medicine for diabetic patients. Humanized insulin was the first biologics drug and became available in 1982 following the advent of biotechnology. It was also a key milestone marking a new era in the pharmaceutical industry.
Following insulin, there has been a flood of antibodies that were developed and become key products as therapeutics. A good example will be Humira which was developed by Abbvie and approved in 2002. Over the last 20 years, Humira has been a blockbuster drug and was the top-selling drug for many years. In recent years, other blockbuster antibodies or antibody-type drugs are Eylea (Regeneron, Sanofi), Keytruda (Merck), and Dupxient (Regeneron, Sanofi).
Looking ahead into the future, bispecific antibodies and antibody-drug conjugates are expected to see strong growth. Bispecific antibodies are drugs that bind to two different targets. The below image shows the different use cases of bispecific antibodies.
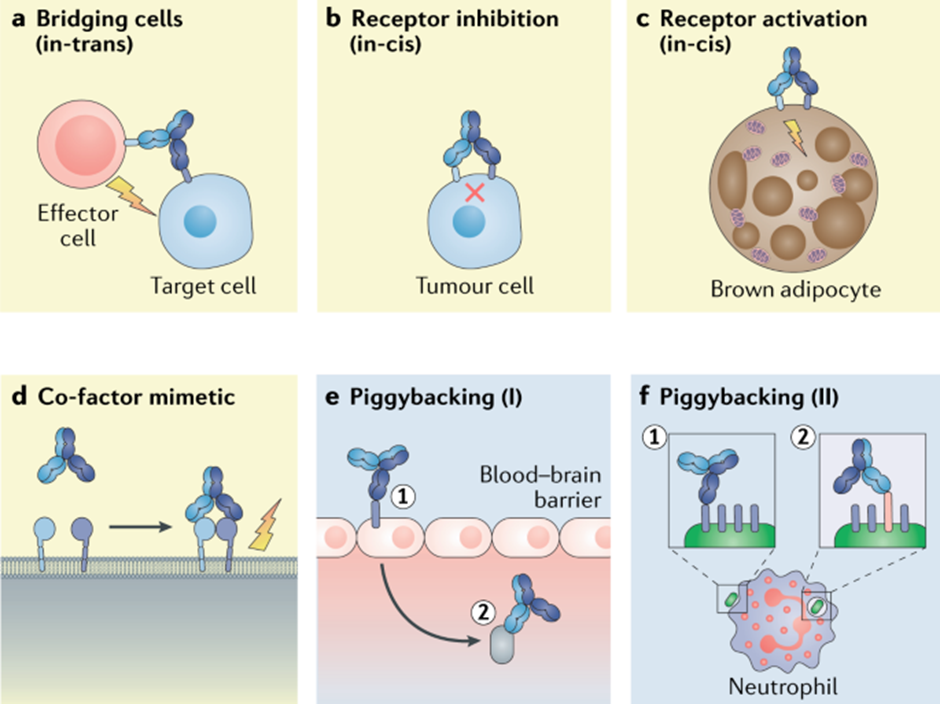
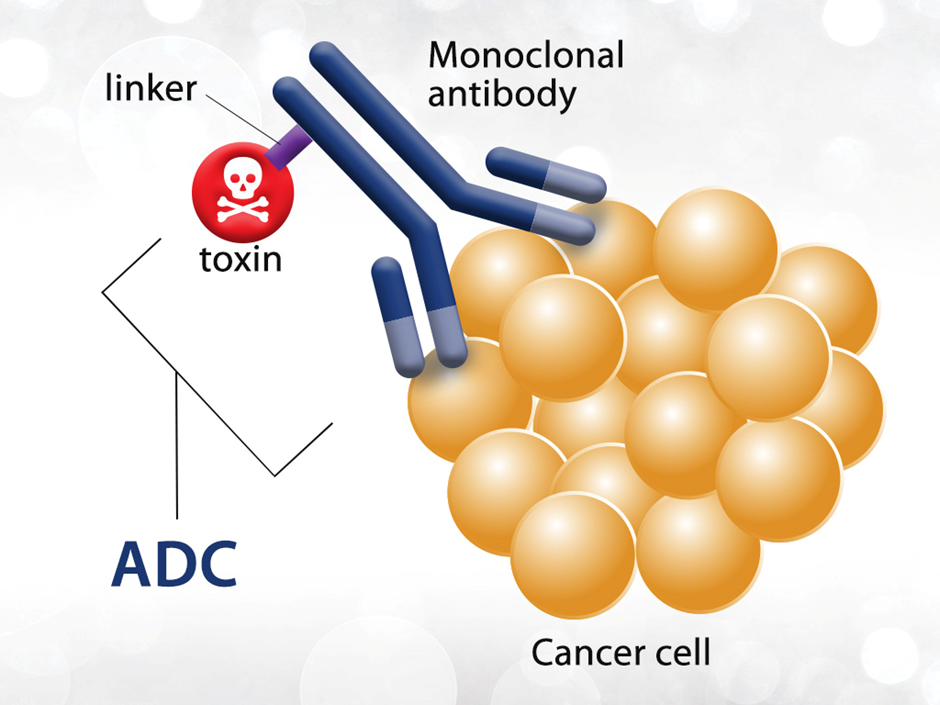
Antibody-drug conjugates are antibodies that are linked to small-molecule drugs or toxins. The antibody part of the molecule allows for targeting and specificity thereby increasing the concentration of the drug at the required site. The increased specificity also reduces potential side effects from systemic dosing of highly toxic molecules which are often used in cancer therapy.
3) Cell & Gene Therapy
Cell & Gene therapy is a broad set of therapies that fall into either: Gene Therapy or Cell Therapy
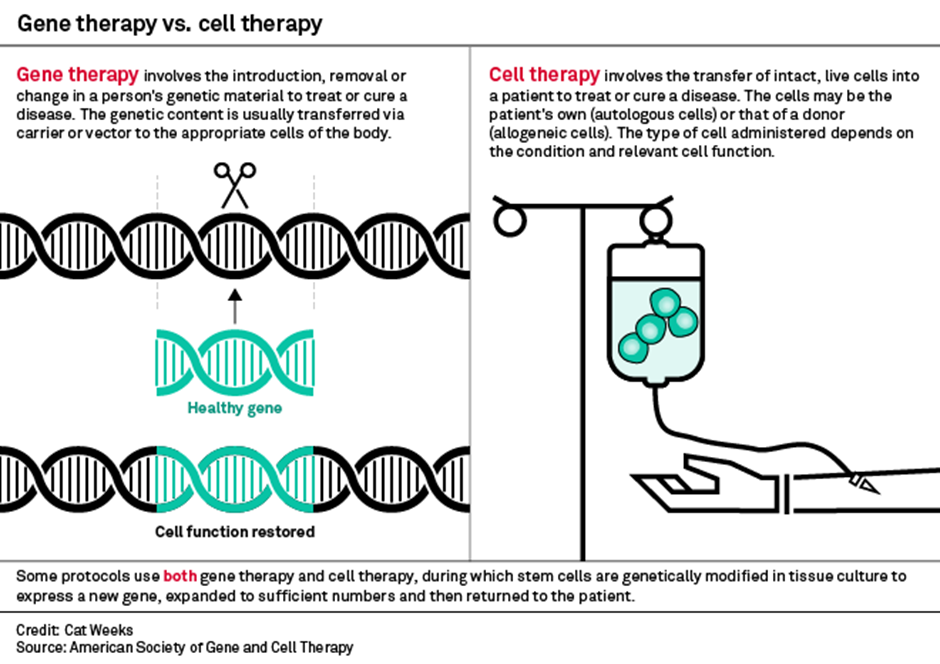
Let us first look at Gene Therapy.
Gene Therapy is a technique that introduces/modifies a person’s DNA to treat or cure disease.
While the first Gene therapy was used as a therapeutic in the 1990s but was eventually halted when a second gene therapy trial led to a patient's death. The patient passed away due to the immune system being overwhelmed by the virus vector that was used to introduce the gene into the patient.
Following the death, Gene Therapy took a back seat, and researchers and companies worked to improve its safety. Then in the 2000s with the discovery of CRISPR/CAS9, gene therapy made its comeback.
With improved knowledge and technology, gene therapy is making a comeback with a couple of approved therapies and many more in clinical trials.
Examples of approved gene therapies include – Gendicin, Luxturna, and Zolgensma.
Using Zolgensma as an example, the drug is an adeno-associated virus (AAV) vector-based gene therapy indicated for the treatment of pediatric patients less than 2 years of age with spinal muscular atrophy (SMA) with bi-allelic mutations in the survival motor neuron 1 (SMN1) gene.
In simple language, this means that Zolgensma helps to introduce a functional SMN1 gene into the individual because his genes are mutated or not functional. The graphic below explains this.
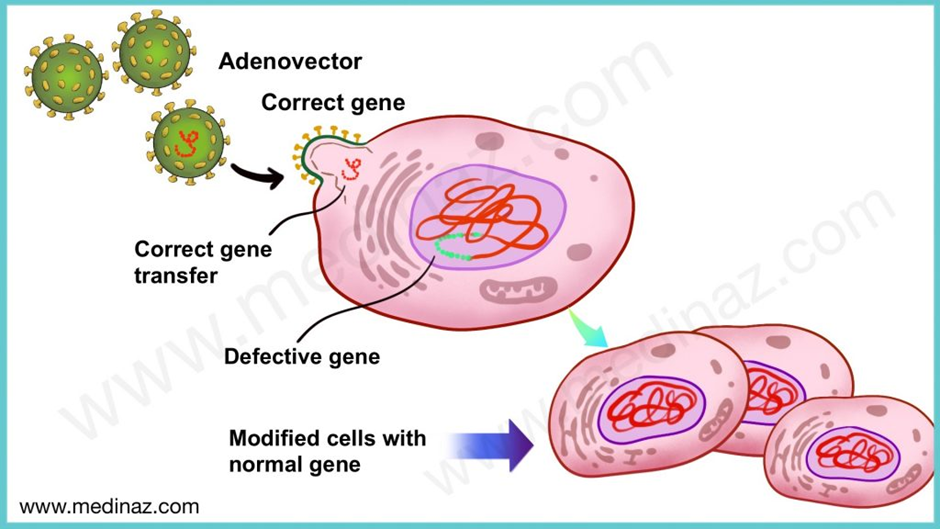
Other gene therapies in trials include those that allow for the editing of genes using the CRISPR CAS9 technology or its derivates. We wrote an article explaining the different CRISPR technologies and highlight eight gene therapy companies, you can read it here.
A full list of FDA-approved gene and cell therapies can be found here.
Moving on to cell therapies, Cell therapy is a technique in which whole cells are infused into the body.
There are various types of cell therapies such as T Cell therapy, Stem Cell therapy, B cell therapy, and others. So how are cells infused into the body? Let’s look at the process quickly.
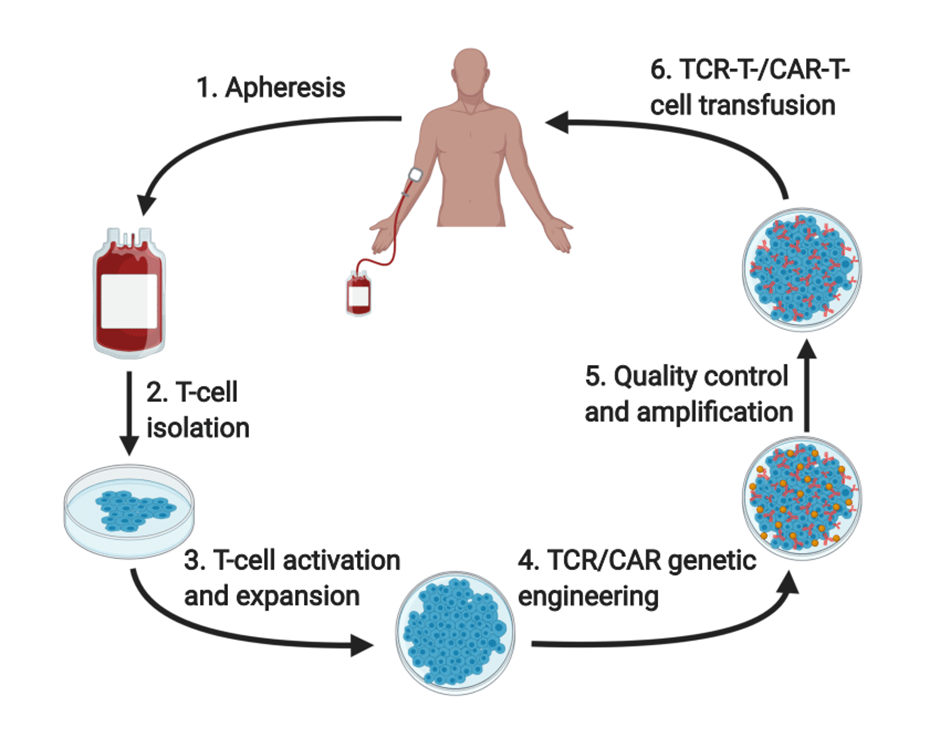
For T Cell therapies, cells are removed from a patient's body, genetically modified, and then re-infused into that patient's body. This is called Autologous (own cells) cell therapy. The process can be seen in the above graphic.
More recently the first allogeneic T-Cell therapy was approved. For these therapies, the process is mostly the same with the key difference being that cells are not removed from the patient but collected from a donor. The cells are then genetically modified and infused into the patient.
There are multiple Autologous T cells therapies not approved with Yescartas by Gilead reporting quarter-over-quarter increases in sales. This shows the high efficacy of the therapy and why it is a go-to for many physicians. Yescartas was first approved as a third-line therapy but is slowly moving up the ranks. It is likely to be first-line therapy in a few years times.
Like T Cells, other cell therapies using stem cells, B cells, and NK cells are either approved or in development.
4) RNA Therapy
RNA Therapies are designed to correct a mistake, or mutation, in the RNA of someone with a genetic disease without changing the DNA itself.
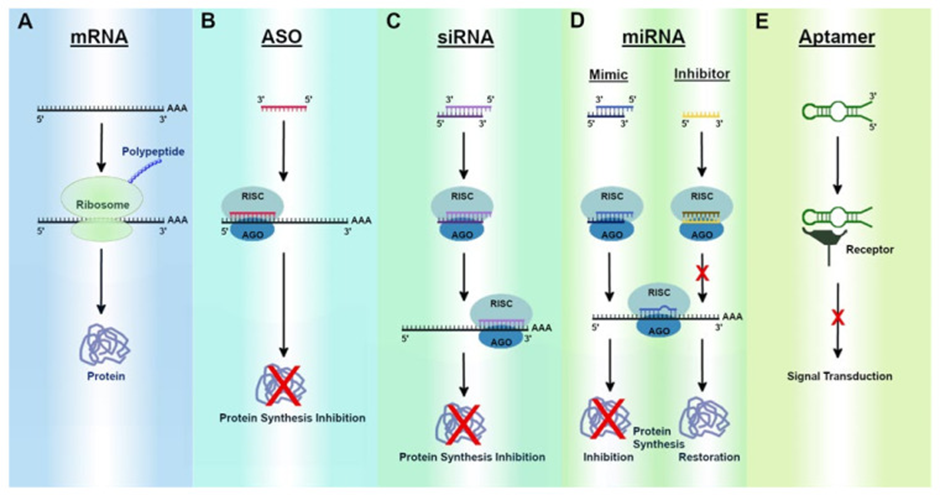
Most RNA therapies can be sorted into one of three broad categories (See image below for visuals):
1) those that target nucleic acids (either DNA or RNA), which can be further divided into two distinct types of therapies:
a. Single-stranded antisense oligonucleotides (ASOs) and,
b. Double-stranded molecules that operate through a cellular pathway known as RNA interference (RNAi)
2) those that target proteins (aptamers), and
3) those that encode proteins (mRNA).
Also, hybrid approaches that combine several RNA-based mechanisms into a single package are emerging.
The first RNA therapeutic was approved in 1998. Since then there have been multiple RNA therapeutics gaining approval.
To get a full understanding of RNA therapeutics, we refer readers to our previous post in which we cover RNA therapeutics in detail, you can read it here.
It’s a wrap.
We looked at four different drug modalities which make up the bulk of the approved therapies out there. While small-molecule drugs have been around for a long time and were the pillars of traditional medicine, in the last two decades drugs have seen a wave of innovation. This was led by biologics, which found its footing when insulin was successfully expressed and biologics now make up almost 50% of newly approved therapies. RNA, gene therapy, and cell therapies are newer modalities that are expected to see strong growth in the future as scientists continue to develop new therapies and approaches.
If you are reading this, it probably means you enjoyed reading the post. We suggest you hit the subscribe button so you do not miss out on any of our future posts. Click here to Subscribe now.
Disclaimer: All opinions shared in this article are the opinions of the authors and do not constitute financial advice or recommendations to buy or sell. Please consult a financial advisor before you make any financial decisions.




Comments ()